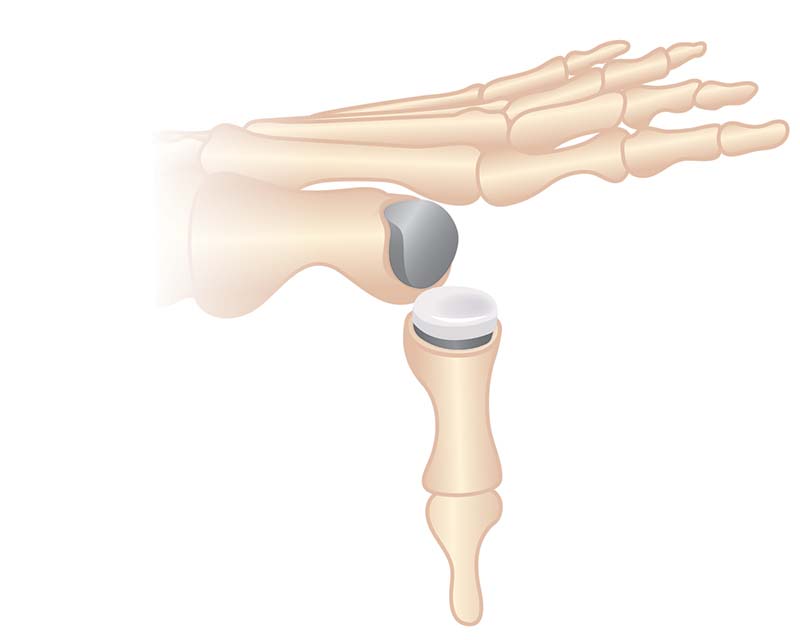
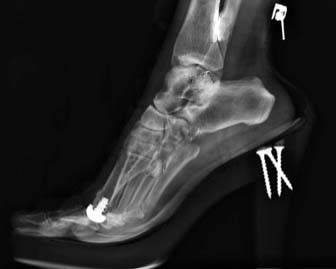
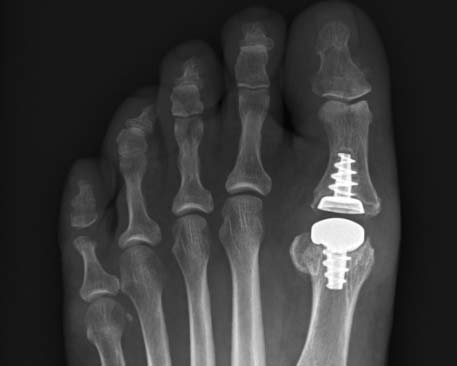
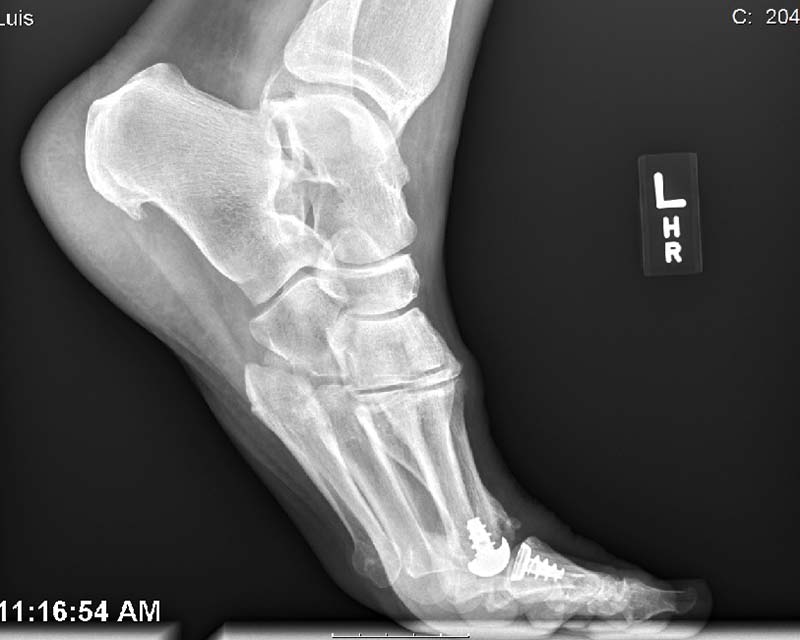
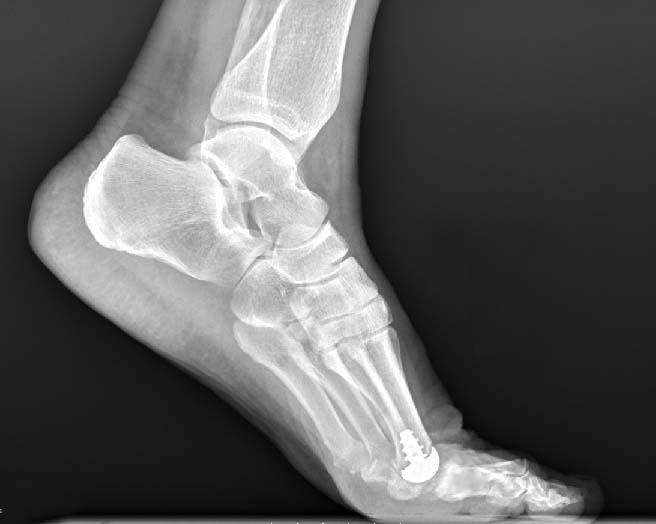
Anika’s Toe HemiCAP® implant system is a minimally invasive metatarsal implant that matches the shape and contour of each individual patients’ joint while protecting the remaining healthy cartilage. This Toe hemiarthroplasty implant removes minimal bone and maintains range of motion by incorporating an extended dorsal curve (Dorsal Flange) into the design intended to improve dorsal roll-off while preventing osteophyte regrowth. The Toe HemiCAP DF is comprised of two separate components, a cap and screw that mate together via morse taper lock unlike one-piece synthetic and stemmed toe implants that have been shown to loosen or slip.
Key Features & Benefits
- Custom matched to fit a patient’s joint size and shape
- Significantly less cartilage and bone are removed than traditional joint replacements
- Range of motion is preserved
- System respects sesamoid tracking
- Anatomic design preserves native structures
- No bridges burned for future surgical intervention
ToeMotion Implant System
Anika’s total toe arthroplasty system restores mobility and maintains the native biomechanics of the 1st MTP joint. This total toe implant is different than other replacements because it is implanted into the bone rather than on top, leaving natural bone to support the implant around its edges. ToeMotion® is also the only total toe replacement that utilizes screws on both sides of the joint to provide rock-solid fixation.
Key Features & Benefits
- Anatomic design fits to a patient’s joint size and shape
- Range of motion is preserved
- System respects sesamoid tracking
- Anatomic design preserves native structures
- Screw fixation provides a stable implant
- Maintains length of metatarsal
- Proven clinical history